Silver fur demonstration
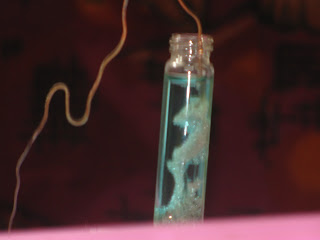
Have u seen Ag-fur, i.e., fine deposits of Ag. Easy just take a concentrated solution of AgNO3 and dip a Cu wire into it. Ag will be displaced by Cu (E Ag = 0.80 V, E Cu = 0.34V). The Cu will get oxidised to Cu2+ ion which will go to the solution and Ag+ ions will be reduced to Ag which will be deposited on the cu wire. The Ag deposits will appear as a beautiful garland of fur. The solution will turn from colourless to blue and finally when u remove the Cu wire it becomes as thin as a hair. I often demonstrates it to my students during electrochemistry classes to give idea about electrode potential. Just go through the snaps taken by my students
1. Silver nitrate solution
2. Cu wire immersed into it, crystals of silver starts depositing
3. Solution slowly turns blue as copper goes into the solution
4. Ag fur grows
5. Ag garlanded the cu wire
6. Ag deposits seen after the cu wire is removed
7. The beauty of Ag manifest itself
8. Me with the Ag fur, a precious moment
For Photgraphs thanks to my HS 2nd Year Chemists







No comments:
Post a Comment