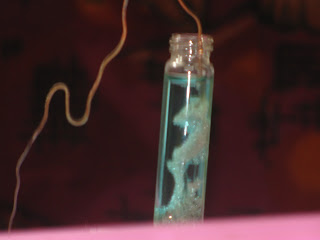
MY DANIEL CELL
You can construct your own Daniel cell and try to increase its voltage by changing the design and composition. I am sharing my Daniel cell which yeilded 0.52V at the very first attempt.
Here is the lay out:
What you need:
1. Two beakers or glass
2. A small plastic tube
3. Cu wire or plate
4. Zn wire or plate
5. CuSO4
6. ZnSO4
7. KNO3
8. Connecting wire
9. A Voltmeeter
What u have to do:
Immersed the Cu wire or plate in copper sulphate solution taken in a beaker and the Zn plate or wire in zinc sulphate solution taken in another beaker. Fill the plastic tube with KNO3 soltion and plug the ends with cotton. This will be your salt bridge. Place the salt bridge in the two beakers like a U-shape bridge . Your Daniel cell is ready. Connect the two terminals to the probes of a multimeter or voltmeter. Measure the volt produced.